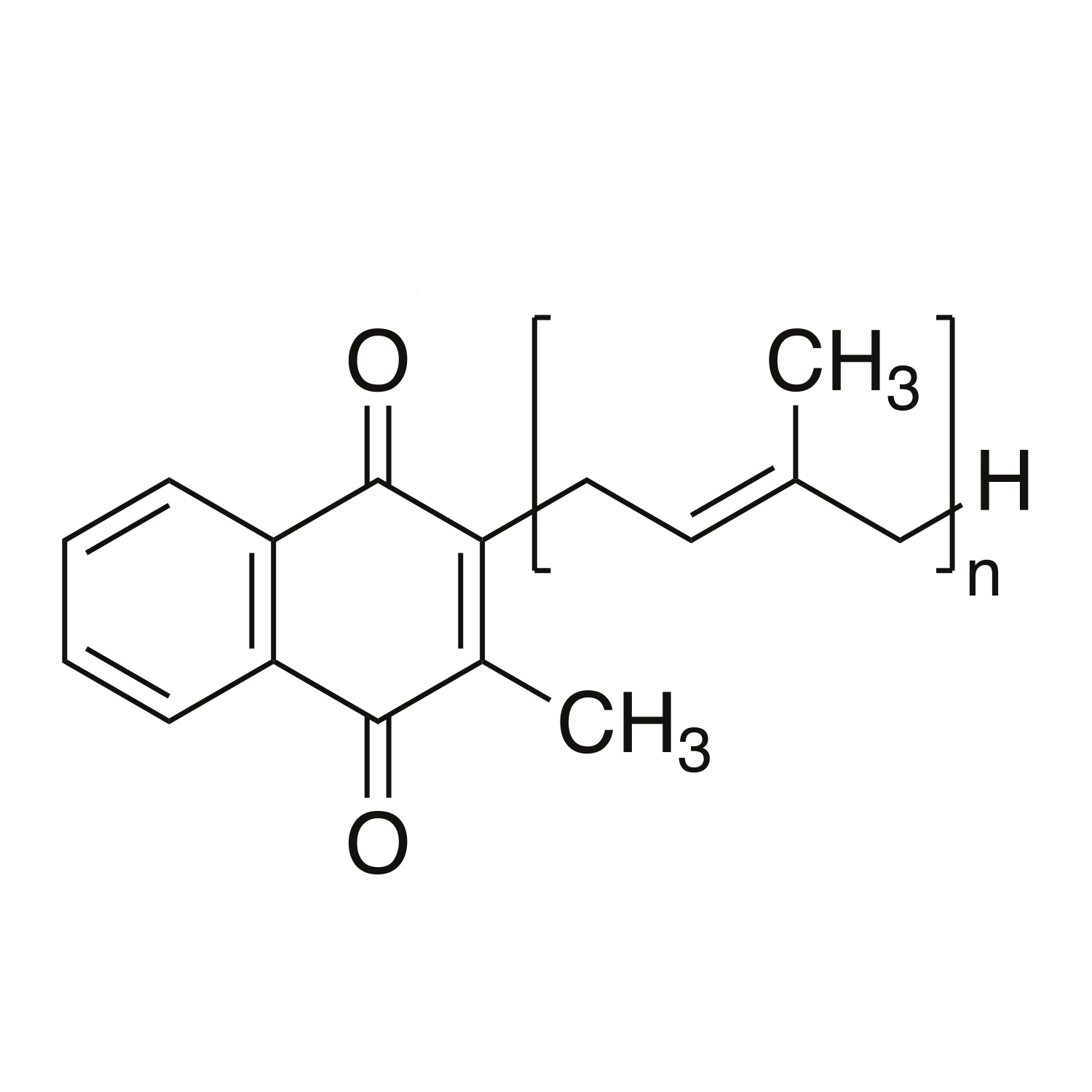
Vitamine K2 in de gepatenteerde vorm K2VITAL® DELTA. In K2VITAL® DELTA is de goed opneembare vitamine K2-MK7 dubbel ingekapseld voor complete stabiliteit. K2 bestaat verder uit alfalfakruid en rijstmeel. Dit supplement bevat geen synthetische vul-, hulp- of kleurstoffen (Clean Label) en heeft een plantaardige pullulan capsule.