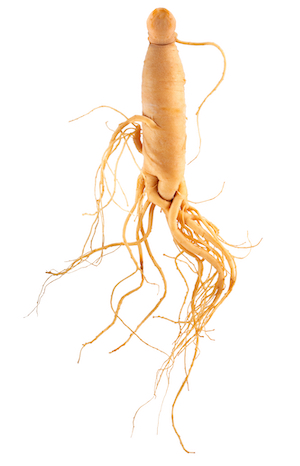
Een combinatie van plantenextracten van Siberische ginseng, ashwagandha en boswellia serrata in een plantaardige pullulan capsule. Siberische ginseng ondersteunt het immuunsysteem*, ashwagandha heeft een gunstige invloed op het uithoudingsvermogen* en boswellia serrata is goed voor het behoud van soepele gewrichten*. We hebben Power ontwikkeld om sporters te ondersteunen, maar zoals je ziet is het een product dat voor iedereen geschikt is.